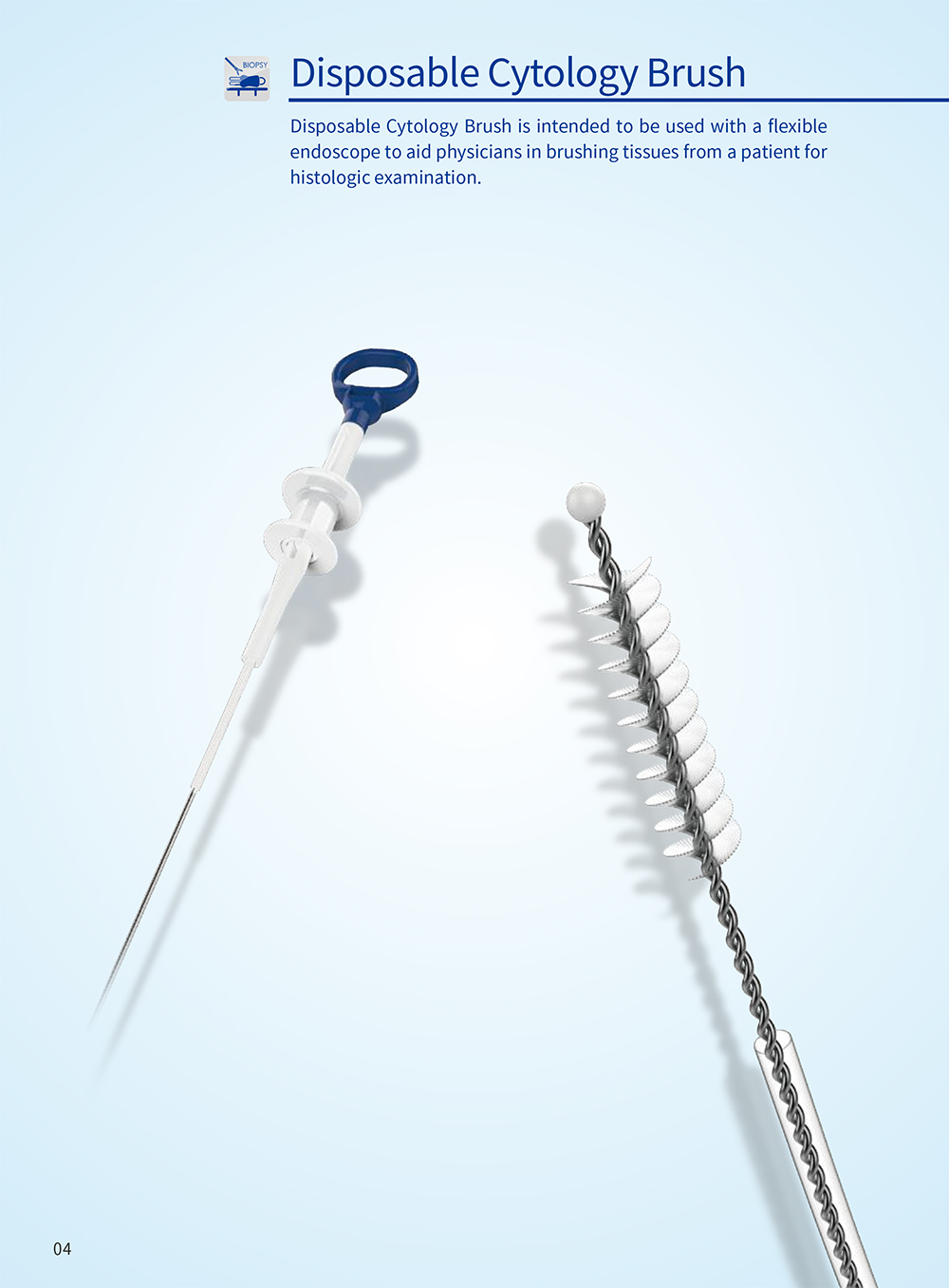
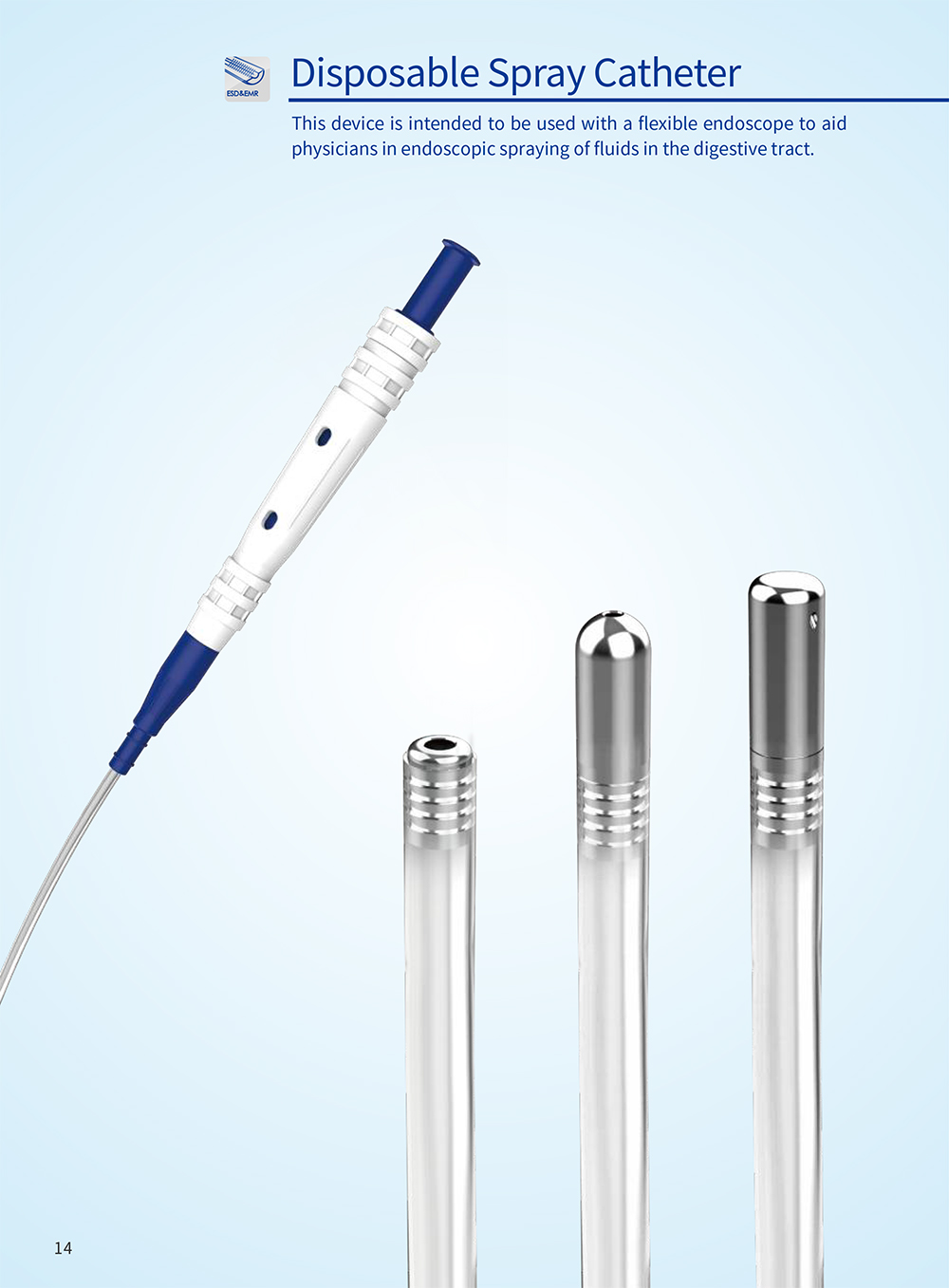
产品中心
Product Center
More

关于精锐
About Jingrui
Hangzhou Fuyang Jingrui Medical Technology Co., Ltd., established in 2005, is a high-tech enterprise specializing in the production and research and development of endoscopic consumables. As one of the earliest enterprises in the industry to pass the ISO9001, ISO13485 quality management system, and EU CE certification, Jingrui always adheres to providing "professional", "standard", and "high-quality" products as our responsibility, and has won the trust of domestic and foreign medical professionals with our excellent product quality.
Currently, the company has advanced production and testing equipment, a 6000 square meter standardized factory building, a 100,000 level purification production workshop, and a 10,000 level laboratory. We have a complete and mature production line with a designed production capacity of 1.8 million pieces. Additionally, to provide timely and convenient pre-sales and after-sales services, the company has established 15 offices in major cities across the country.
Currently, the company has advanced production and testing equipment, a 6000 square meter standardized factory building, a 100,000 level purification production workshop, and a 10,000 level laboratory. We have a complete and mature production line with a designed production capacity of 1.8 million pieces. Additionally, to provide timely and convenient pre-sales and after-sales services, the company has established 15 offices in major cities across the country.

发展历程
Development history
In 2021, JINGRUI’s parent company Aohua Endoscopy Shanghai was successfully listed.
In 2019, all products get the CE certificate--Development in an all-around way
In 2018, Became a Class III medical device manufacturer and opened up the Japanese and South African markets. --Business expansion
In 2016, Strategic Partnership with AOHUA Endoscopy Shanghai-- Complementary of strengths
In 2015, Moved to the current 6000 sqm new factory building--Entirely new complexion.
In 2005, company founded, and already has 18 years of experience--Well tested
Contact us
Add: No.99, Waizhougongwu, Guanqian Village, Yinhu Street, Fuyang District, Hangzhou City, Zhejiang Province
Tel: +86-571-63417238/63417239
E-mail: yao.huifen@aohua.com zhao.guizhen@aohua.com accessories@aohua.com

COPYRIGHT (©) 2023 Hangzhou Fuyang Jingrui Medical Technology Co., LTD make from ZJHZ
COPYRIGHT (©) 2023 Hangzhou Fuyang Jingrui Medical Technology Co., LTD make from ZJHZ
 杭州富阳精锐医疗科技有限公司
杭州富阳精锐医疗科技有限公司